NeuroSENSE® OEM Module
(Under Development)
ProductDescription
The NeuroSENSE OEM Module was designed to provide third-party patient monitors with the benefit of depth-of-anesthesia or sedation monitoring in an economical and rugged package. Powered directly through its serial interface, the device provides its host with high resolution bilateral EEG signals and the NeuroSENSE processed variables. The NeuroSENSE OEM Module is specifically designed to be used in conjunction with the Electrode Kit EK-901 for the utmost signal quality.
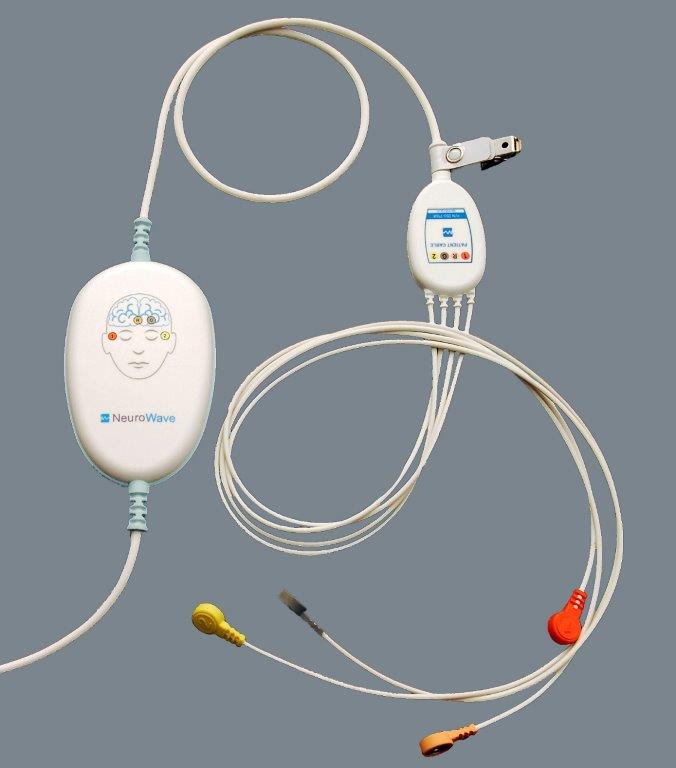
ProductHighlights
- Compliant with the IEC 80601-2-26 standard
- High level of patient electrical isolation
- Protection against cardiac defibrillation
- Continuous electrode impedance measurement
- Automatic detection of lead disconnection
- Utilizes NeuroWave’s patented electro-surgical knife interference filter and detection circuitry
- State-of-the-art low noise pre-amplifier electronics
- Processed Variables:
- Bilateral WAVCNS (with automated trending), Suppression Ratio (SR), and Electromyogram (EMG) parameters
- Artifact flag, including ocular activity and electro-cautery interference detection
- Signal quality measures, including continuous electrode impedance and environmental noise
- Specifications:
- Power consumption: 0.8 W
- Interface protocol: USB 2.0 and above
- Electrode compatibility: EK-901
